It has been a long time since a new therapeutic has been approved to help treat sickle cell disease (SCD) and its associated complications. People who suffer from the debilitating disease will be hoping for positive outcomes for the candidate drugs, which are now in or approaching late-stage clinical trials. One of those is vepoloxamer from San Diego-based Mast Therapeutics Inc., which anticipates reporting top-line data from a phase III study of the compound in 388 SCD patients this month.
The results from the EPIC study of vepoloxamer (MST-188), a purified form of a nonionic, triblock copolymer (poloxamer 188), will be released a little later than originally planned, but the company said the delay does not reflect on the quality or integrity of the results or conduct of the study but rather factors related to validation of the data points and quality assurance/quality control procedures, which have taken longer than anticipated.
Vepoloxamer is designed to lower the viscosity of SCD patients' blood during a crisis by lowering adhesion and improving the malleability of cells, improving blood flow and thereby shortening the duration of the crisis.
Confidence
In a research note, Piper Jaffray analyst Edward Tenthoff said "prior phase II data and enrollment stats increase our confidence of success" of the trial. It has been designed to show a 16-hour reduction with vepoloxamer vs. saline control on top of standard of care in the primary endpoint of duration for vaso-occlusive crisis (VOC) as measured from the last dose of I.V. pain medication. It is estimated that VOCs are responsible for more than 70,000 hospitalizations of SCD patients per year in the U.S., which require them to be hospitalized for an average of about six days.
Vepoloxamer has the potential to reach the market in 2017, if the late-stage testing proves successful. U.S. sales have been forecasted to be more than $400 million by 2023, according to Piper Jaffray.
Emmaus Medical Inc., a specialty pharmaceutical and subsidiary of Emmaus Life Sciences Inc., completed a 230-patient phase III trial on levoglutamide (L-glutamine) in December 2013 and said it is preparing its new drug application for the oral compound to treat some of the symptoms of SCD.
Results from other advanced studies are not scheduled for another couple of years or more. For example, rivipansel (GMI-1070), from Glycomimetics Inc., of Gaithersburg, Md., an investigational pan-selectin inhibitor designed to improve the flow of blood and reduce the duration of pain, has also entered late-stage testing. Selectins are a family of molecules believed to play a key role in regulating cellular interactions within blood vessels. Last year, the company's partner, Pfizer Inc., started the RESET (Rivipansel: Evaluating Safety, Efficacy and Time to Discharge) study – a phase III trial assessing the efficacy and safety of the compound for the treatment of VOC in hospitalized SCD patients, ages 6 or older. The study is planning to enroll at least 350 people.
Pfizer entered a worldwide license agreement with Glycomimetics for the development and, if approved by applicable regulatory authorities, commercialization of rivipansel. Glycomimetics was responsible for development up to phase II, and Pfizer will now be responsible for all future clinical development. (See BioWorld Today, Oct. 12, 2011.)
The pharma company is building a growing franchise in SCD. Its PDE 9a inhibitor (PF-04447943) has entered a phase Ib study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of the compound when co-administered with hydroxyurea in patients with stable SCD. Completion of that trial is estimated to be late July.
Product development
Although many other potential SCD therapies under development have yet to reach phase III testing, patients will be encouraged by the fact that the pipeline is expanding as technologies such as gene therapy uncover promising new therapies to treat the disease.
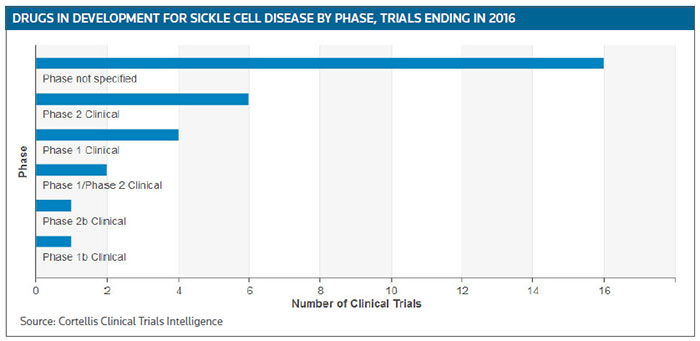
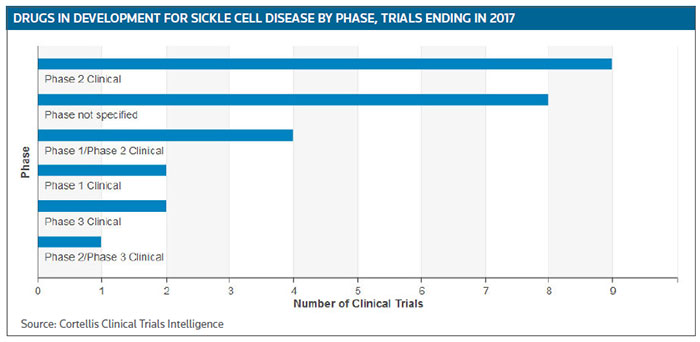
For the rest of this year and into 2017 about 15 phase II trials are scheduled to be completed, or have already have been completed, according to Cortellis Clinical Trials Intelligence. (See Sickle Cell Disease clinical trials targeted for completion in 2016 and 2017, below.)
In June, Global Blood Therapeutics Inc., of South San Francisco, reported results from its ongoing phase I/II GBT440-001 study in SCD at the European Hematology Association meeting in Copenhagen, Denmark, including a presentation of 90-day data from a cohort of patients taking 700 mg/day of GBT440. The data showed a durable reduction in hemolysis from baseline, as evidenced by a rapid and sustained reduction in bilirubin starting as early as day four. Results also showed a median 1.1g/dL increase in hemoglobin concentration with GBT440, compared with a 0.2 g/dL decrease with placebo, and a median decrease of about 20 percent in reticulocyte count compared with a roughly 20 percent increase with placebo.
Twenty-eight-day results from three dosing cohorts of GBT440 showed a therapeutic target between 10 percent and 30 percent hemoglobin modification achieved at doses of 500 mg or greater, with a consistent beneficial effect seen it at least one key parameter: hemolysis, reticulocytosis or sickle cell counts. Pharmacokinetic and pharmacodynamic data confirm the mechanism of action and support once-daily dosing, the company said. GBT440 is believed to work by blocking polymerization and the resultant sickling of red blood cells.
Cowen and Co. analyst Ritu Baral commented, "We are highly encouraged by not just the magnitude of the median Hb increase (1.1g/dL), but also the consistency of hemolysis marker (reticulocyte and bilirubin) improvement and the compound's excellent safety data."
The company has also initiated a phase IIa study of GBT440 in adolescents, ages 12 to 17, with SCD. The study is being conducted in two parts: In part A, six subjects will receive a single dose of GBT440 and, in part B, 24 subjects will receive multiple doses of GBT440 for up to 28 days. The primary objective of part A is to characterize the pharmacokinetics, and for part B to explore the safety of multiple doses of GBT440.
Later this year, Dilaforette AB, of Stockholm, is scheduled to report the results from a phase II study on its polysaccharide compound, sevuparin, which was started in October 2015.
The study in hospitalized SCD patients experiencing VOC will be treated with I.V. infusion of sevuparin or placebo on top of standard pain medication, which is I.V. infusion of opioids given during VOC. It is designed to demonstrate reduced time to resolution of VOC, defined as freedom from parenteral opioid use and readiness for discharge from hospital.
Dilaforette is working with Ergomed plc under a co-development deal, which sees that firm co-invest a proportion of its revenues from the clinical and regulatory activities of that trial in return for an equity stake in Dilaforette.
Selexys Pharmaceuticals Corp., of Oklahoma City, is conducting the SUSTAIN trial, a phase II study in 174 patients to assess safety and efficacy of the anti-P-selectin monoclonal antibody SelG1, with or without hydroxyurea therapy, in SCD patients with related pain crises. Patients are randomized to receive high-dose SelG1, low-dose SelG1 or placebo in the presence or absence of hydroxyurea therapy. The study will examine the effectiveness of SelG1 in reducing the rate of sickle cell-related pain crises in each active dose level as compared to placebo. Results are also expected this year.
In preclinical studies, inhibition of P-selectin has been shown to effectively prevent vaso-occlusion by blocking critical cell-cell interactions that drive that process. Therapeutic blockade of P-selectin, the company believes, may therefore reduce or prevent VOC in patients with SCD.
Certainly, the medicine chest for SCD therapies looks as though it will be replenished over the next five years, with more than 60 compounds now being tested in mid- or late-stage trials, according to Cortellis Clinical Trials Intelligence.