Building a successful biotechnology company requires that its executives make decisions to help navigate a smooth path around barriers that could easily hinder progress. There is no doubt that running a biopharma company involves plenty of risks in an ever-changing financial and regulatory landscape.
Two recent surveys provide insights into what management believes are the key impediments to their growth and what strategies they are developing in order to help mitigate the risks they continually face.
In one survey, 254 global biopharma executives were polled in March on risk management in the light of their current and future business operations. Specifically, it was designed to discover how their companies intend to manage new risks associated with the current biopharma business environment as they build out their therapeutic pipelines and expand their operations both domestically and globally.
Sponsored by MilliporeSigma, and conducted by the Economist Intelligence Unit (EIU), the Changing Biopharma Risk Equation report describes that regulatory uncertainty topped the list of risks that biopharma executives think might disrupt their company's strategy in the next five years (32 percent); a lack of funding for growth was the second biggest concern (25 percent) for respondents overall.
The regulatory impediment could stand in the way of expansion into new products, expansion into new types of therapeutic categories and expansion within existing and into new geographic markets, which survey respondents overwhelmingly indicated are their top strategic goals for growth for the next five years.
The survey found that stem cell-derived therapies and gene therapies are at the top of the list of drug categories deemed likely to disrupt short- and long-term corporate strategies. Almost half (48 percent) of respondents indicated that they are developing or will develop therapies in that space.
The survey found that companies are looking to expand globally over the next five years, with emerging markets being the main focus. Firms are also confident, with 80 percent of respondents reporting that they are highly optimistic about their company's ability to bring new drug products to market over the next five years, a finding that is consistent regardless of company size.
Drug pricing: 'elephant in the room'
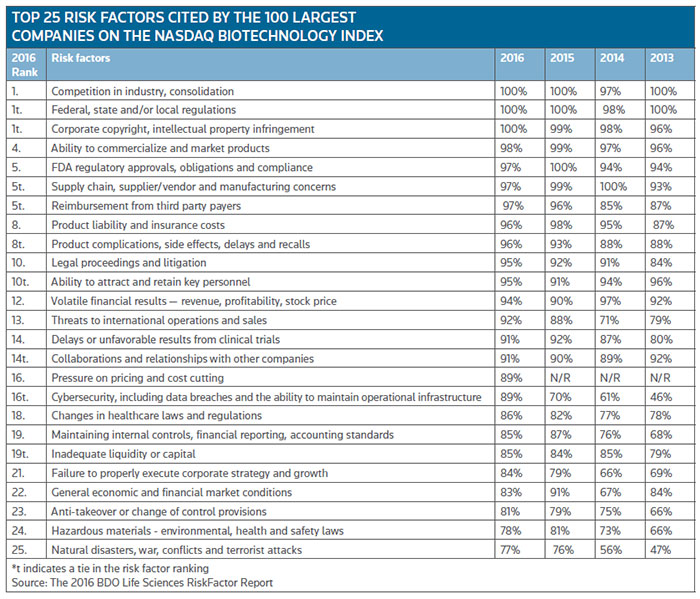
There is no doubt that the thorny issue of drug pricing continues to hang over the life sciences sector, with nearly nine in 10 (89 percent) of organizations identifying drug pricing pressures as a considerable risk, according to the 2016 BDO Life Sciences RiskFactor report published by accounting and consulting organization BDO USA LLP. It's BDO's fourth report to examine the risk factors cited in the most recent annual shareholder filings of the 100 largest publicly traded U.S. companies listed on the Nasdaq Biotechnology Index by revenue. The risk factors were analyzed and ranked in order of frequency cited, Ryan Starkes, assurance partner and leader of the Life Sciences Practice at BDO, told BioWorld Insight.
From the analysis, it looks as though for the near term "drug pricing remains the elephant in the room," Starkes observed.
That is the first time the report has tracked the drug pricing risk factor separately and notes that due to its lingering effect coupled with geopolitical uncertainties, "the road to growth may not be a straight one."
The top three risks among the top 25 risk factors identified this year are: industry competition and consolidation, regulatory hurdles and intellectual property (IP) infringement. (See Top 25 risk factors, below.)
All 100 companies researched in the analysis cite regulatory concerns, with 86 percent calling out changes in health care laws and regulations — up 4 percentage points from the previous year.
They also found that 97 percent cite reimbursement from third-party payers, including payments from Medicare and Medicaid, as a risk factor. That may be explained by the shift from fee-for-service to value-based care, which is forcing companies to re-evaluate how they price their products.
High on the list of risk factors, companies identify volatile financial results, including stock prices, as a key threat (94 percent). Concerns around debt also emerged at the forefront of the industry's risk factors this year, with 71 percent of companies citing indebtedness as a risk — up 15 percentage points from 2015.
Cyber Threats Becoming a Major Risk
A significant number of companies cited cyber risk in their annual filings, up 19 percentage points from 2015 and 43 percentage points from 2013. The report explains that life sciences companies have access to extremely valuable data assets, which can translate into big payouts for cybercriminals. "The biggest cyber threat in the life sciences industry is arguably to its intellectual property, which may fall victim to insider theft or corporate espionage."
All of the companies analyzed cited risks related to IP and potential infringement of competitors' patents, and subsequent litigation is also a major concern, with 95 percent citing legal proceedings and litigation as risks in their annual filings. Loss of patent exclusivity (60 percent) is also a major concern.
Not surprisingly, companies are worried about their product development. The vast majority cite delays or unfavorable results from clinical trials (91 percent) and FDA regulatory approvals (97 percent) as significant risks.
Although dealmaking has increased significantly, the report found that three-quarters of companies cite the inability to manage, complete and integrate current or future transactions as a risk in their annual filings.