The biopharma sector has taken advantage of renewed interest from investors and welcoming financial markets, with 25 companies focused on developing new medicines successfully completing their IPOs on U.S., European and Australasia exchanges in the first half of the year – 20 percent more than the total who graduated to the public ranks this time last year. The amount raised to date is also almost twice as much generated from IPOs last year. (See Amount raised from biotech IPOs by regional listings, below.)
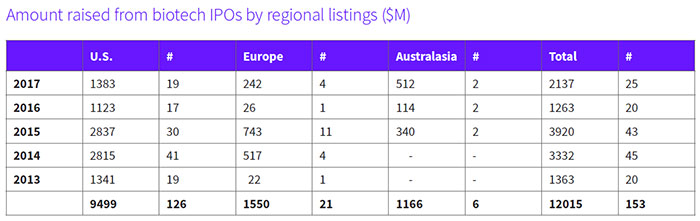
The statistics for biotech IPOs are in keeping with the experience of most sectors, with Renaissance Capital reporting that in the U.S. the appetite for IPOs appeared to pick up momentum in the second quarter, with 52 IPOs being completed, raising almost $11 billion, a two-year high in terms of deal count and proceeds. In fact, the 2017 U.S. IPO market so far has raised more capital than in all of 2016. Technology and health care companies accounted for about half of the 77 IPOs completed in the first half of the year.
At the close of the second quarter, this year's crop of new public biopharma companies had generated $2.1 billion, with those companies listing on U.S. exchanges bringing in almost $1.4 billion for an average of $74 million per IPO. Not surprisingly, 63 percent of those companies are focused on cancer therapies. Among them is Cambridge, Mass.-based Jounce Therapeutics Inc. (NASDAQ:JNCE), which closed an upsized offering of 7.3 million shares at $16 each, above the intended range of $13 to $15, for proceeds of $117.1 million, which included the full exercise of the underwriters option to purchase additional shares. (See BioWorld Today, Jan. 30, 2017.)
The company is focused in the hot immuno-oncology (I-O) field, and JP Morgan analyst Cory Kasimov said the company has a strong translational science platform. Its lead asset, JTX-2011, is a monoclonal antibody that binds to and activates the inducible T-cell co-stimulator (ICOS), a protein on the surface of certain T cells. JTX-2011 is intended to treat solid tumors as a single agent and in combination with other therapies. It is currently being evaluated in a phase I/II trial. Data from the phase I portion of that ICONIC (ICOS AgONist Antibody for Immunotherapy in Cancer Patients) study were presented at ASCO 2017. The preliminary data show JTX-2011 to be well-tolerated in doses up to 0.3 mg/kg as a monotherapy and in combination with Opdivo (nivolumab, Bristol-Myers Squibb Co.) in patients with advanced solid tumors.
Beyondspring Inc. raised about $54.3 million from its IPO. It is developing plinabulin, a triple-targeting cancer agent with a mechanism of action that allows for growth inhibition and triggering of apoptosis in malignant cells. In April, the New York-based company enrolled the first patient in its phase II/III study of lead asset, plinabulin, for the prevention of neutropenia, in combination with docetaxel.
Since its debut, the company's share value (NASDAQ:BYSI) has doubled. (See Performance of 2017 U.S. biotech IPOs, below.)
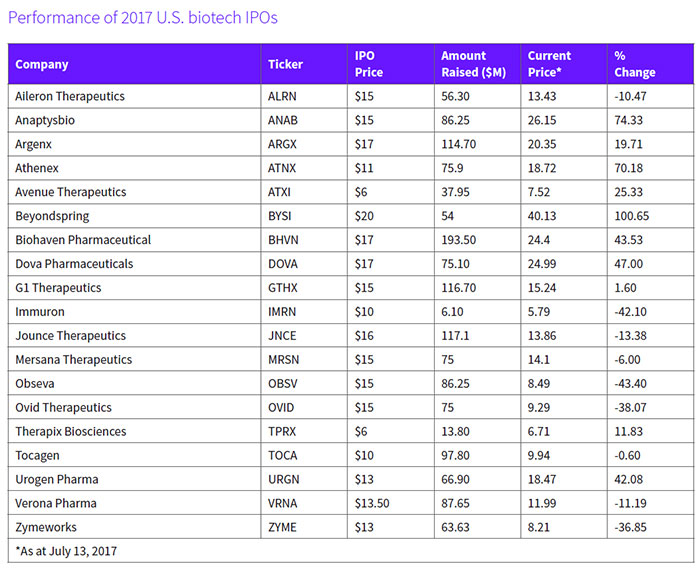
Another cancer-focused company, Buffalo, N.Y.-based Athenex Inc., has seen its shares post a 70 percent jump in value (NASDAQ:ATNX) since its market debut. The company sold a total of 6.9 million shares of its common stock, including 900,000 shares of common stock issued upon the exercise in full by the underwriters of their option to purchase additional shares at $11 per share.
In May, Athenex, along with its partner, Guangzhou Xiangxue Pharmaceutical Co. Ltd., received the Chinese FDA investigational new drug approval to begin clinical trials for KX-02 tablet for glioblastoma. The compound is a new Src protein tyrosine kinase inhibitor and tubulin polymerization inhibitor that can disrupt cancer cell division, induce cell cycle arrest, apoptosis and cancer cell death. The company reported that KX-02 can also cross the blood-brain barrier and has induced durable complete remissions of glioblastoma in human xenograft animal models without sustained therapy.
San Diego-based Anaptysbio Inc. has received favorable investor support, since closing its IPO of 5.75 million shares of common stock at $15 per share. Shares (NASDAQ:ANAB) have soared 74 percent.
The company's anti-inflammatory pipeline includes an anti-IL-33 antibody (ANB-020) for the treatment of moderate to severe adult atopic dermatitis, severe adult peanut allergy and severe adult eosinophilic asthma; its anti-IL-36R antibody (ANB-019) targets the treatment of rare inflammatory diseases.
Enrollment of a phase IIa trial for ANB-020 in adult patients with severe peanut allergy is underway and top-line results from that study are anticipated during the second half of the year. Top-line results are also planned to be released this year from a phase IIa trial of ANB-020 in the treatment of adults with moderate to severe atopic dermatitis.
Good performance so far
Collectively, the 19 newly minted public biopharma companies listing on U.S exchanges this year have returned an average share price increase of 12.3 percent. That performance will certainly encourage other companies now on the IPO runway to launch their offerings in the second half of the year, particularly if the financial climate continues to remain positive.
In its half-year report, Renaissance Capital said the U.S. IPO market is well-positioned for another active quarter. "The extended period of strong IPO returns and low market volatility has created a receptive environment for a wide variety of companies to come public."
According to BioWorld, there are 13 biopharma IPOs who have filed to undertake an IPO, which is low compared to previous years. The low number of filings is not just confined to the biopharma sector. The Renaissance report notes that "the quarter ended with a below-average number of IPOs publicly on file, particularly growth names."
Going forward, the analysts predicted that "biotechs should also contribute to the total deal flow, provided that insiders continue to participate in the offerings."
So far in the third quarter, Akcea Therapeutics Inc., a spinout of Ionis Pharmaceuticals Inc., has priced an IPO, expecting to raise $125 million. (See BioWorld, July 17, 2017.)
Meanwhile, the latest biopharma company to join the IPO runway is Accelerated Pharma Inc., of Westport, Conn., which filed to conduct an IPO of 1.5 million units, to be priced between $4 and $6. In its filing, the company said it is focused on utilizing genomic technology to enhance the development of pre-existing pharmaceutical products for the treatment of various cancer indications, and to identify patients who may respond to such products. Its lead product is picoplatin, a new generation platinum-based cancer therapy that has the potential for use in different formulations, as a single agent or in combination with other cancer agents, to treat multiple cancer indications.

